Brain tumours in children remain a major medical challenge. Not only because of their heterogeneity but because of their tendency to poor prognosis. The appearance of a tumour is often devastating as, for some types, there currently very few effective treatments. While globally around 70% of children diagnosed with brain cancer survive for more than 5 years (according to the CONCORD3 study), in some types of tumour this drops to just 20% (according to the American Cancer Society). There are barely any effective treatments for some of these tumours and the average survival of a child following diagnosis is sometimes little over a year. In addition, the treatments often have to be extremely aggressive and can lead to significant sequelae in patients.
Therefore, methods to improve diagnosis and identify the characteristics of each patient’s tumour are vital in order to apply more specialized and personalized therapies. Brain tumours in children are very different from those that develop in adults and have a number of biological characteristics that mean that they cannot be treated in the same way. If the characteristics or weaknesses can be identified in each child’s tumour and a more appropriate choice of therapy is enabled, we will greatly increase the chances of success and avoid the side effects of therapies that may not even be effective.
Description of the Project:
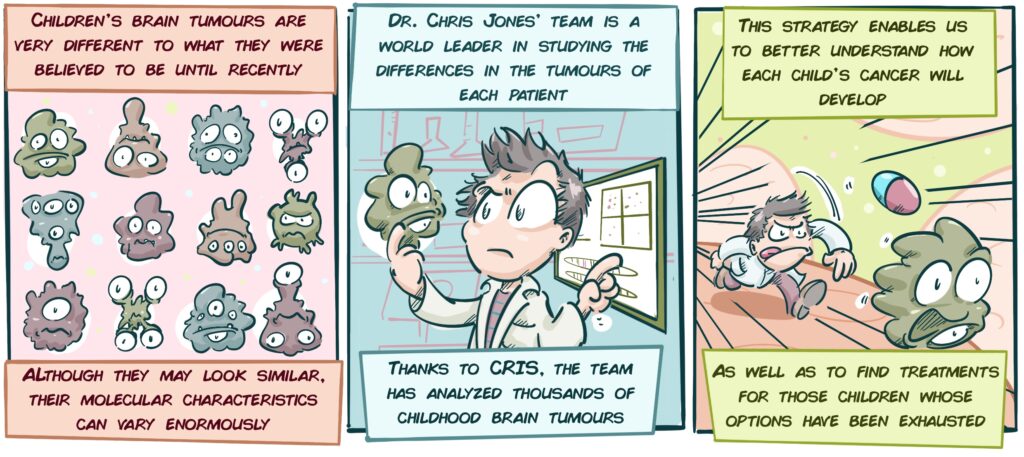
In his work, Professor Chris Jones of The Institute of Cancer Research in London (UK), looks for all kinds of molecular mutations (genetic and all kinds of modifications that occur in cells) in brain tumours to enable us to better differentiate each type of tumour, particularly some of its more aggressive variants, such as Paediatric High-Grade Glioma or Diffuse Intrinsic Pontine Glioma (or DIPG, the tumour type that the CRIS Project on Brain Tumours in France is focusing on). This helps us to better understand the chain of events that occur in a cell as it gets out of control as a pathology develops. Through this, the patient’s prognosis is improved and physicians are able to choose a more appropriate therapy.
An important aspect of this characterization consists of gradually increasing the precision of the classification methods for childhood brain tumours. If patients with tumours of similar appearance and mutations or genetic changes can be grouped together, each group can be treated differently. The more clearly defined these groups, the more appropriate and specific the treatments will be. This group is the frontrunner in Europe for this type of study and almost all of its studies encompass a large number of countries.
This group’s current lines of research include:
- Study of the epigenetic changes of childhood brain tumours: Many of the advances that have been made in the diagnosis and treatment of these tumours relate to genetic analysis, or rather mutations in the DNA of tumour cells. However, sometimes there are non-genetic changes, which relate more to how the cell reads the information contained in the DNA. Studying these changes and combining them with genetic information will enable us to deepen our understanding of these tumours, refine diagnoses and even design new therapies.
- Study of the evolution of childhood brain tumours: The team is conducting a study that will analyze tumour samples from children at various stages in their disease to understand how tumours change over time. This will help us to understand how tumours become resistant to treatments and to anticipate recurrences in future patients.
- Establishment of good laboratory models: To study tumours, understand them and develop therapies, laboratory models that reproduce as closely as possible the behaviour of tumours in real life are essential. In order to do this, Dr Jones’ team is developing a huge collection of 3-D cell cultures, derived from the tumours of a large number of patients.
- Analysis of long-term survivors: Although children with high-grade gliomas or DIPG usually have a very short-term survival rates, some children survive for a long time. Dr Jones’ team is analyzing the characteristics of these patients to understand why they survive and others do not. The idea is to use this knowledge to develop new, better-targeted therapeutic strategies that help children with poor prognoses to survive.
Latest project developments:
Over the past few years, the team has analysed a huge number of aggressive childhood brain tumour samples (high-grade glioma and diffuse intrinsic pontine glioma, DIPG). This has enabled the team to produce a vast catalogue of genetic changes that distinguishes certain tumours from others and explains the behaviour of each. This catalogue, which is public and can be consulted at PedcBioPortal (https://pedcbioportal.org/) forms the basis of much of the team’s current projects.
Determining the changes contained in the cells of each patient’s tumours can save lives: In a study published in 2020, it was observed that patients with Diffuse Intrinsic Pontine Glioma (DIPG) under the age of 4 often have mutations in a protein called ALK2. Through this finding, children with these mutations can be given drugs to fight ALK2, which reduces the size of tumours in some patients. These findings, published in the prestigious Nature Communications journal, add a new weapon to our current meagre arsenal in fighting this aggressive type of tumour.
Until now, most diagnoses of these types of tumours have been based on examining biopsies of these children’s tumours under a microscope. However, as Chris Jones’ team demonstrates in another study published in Cancer Discovery, basing the diagnosis on that method alone can lead to these tumours being inaccurately diagnosed, leading to the incorrect assumption that they are a certain type of brain tumour. This work shows that molecular analysis (genetic and other cellular characteristics) can greatly refine the diagnosis and even identify weak points that can be treated with existing and approved drugs. As has been verified on several occasions by Dr Jones’ group, this is something that can save the lives of children that had run out of therapeutic options.
Regarding the development of 3-D cultures from the brain tumours of patients, the team has been able to build up a collection of over 50 cultures. Benefitting from these laboratory models, they are testing a large number of drugs already approved for use in other pathologies and thus identifying potential new treatments to fight these tumours. The results are very promising and the team has already identified several compounds that could become future treatments against these tumours.
The team is currently focusing on increasing its understanding of the relationship of the immune system on tumour development in children with brain cancer to assess whether immunotherapy could be helpful for any of them.