Introduction:
Sarcomas are tumours that arise from cells that make up the connective tissues that support the body. Round-cell sarcomas, in particular, often affect children and young adults and are known for being very heterogeneous. This always poses a problem since it makes it difficult to study these tumours and the best choice of therapy. It is very important to define and classify the tumours of different patients based on the tumours’ characteristics and weak points because the more we know about them, the easier it will be to identify an appropriate therapy (and, if there is no such therapy, create a targeted therapy).
The best known round-cell sarcoma is Ewing sarcoma, which generally affects the bones and tends to be highly aggressive. Although its characteristics are fairly well defined, even today children with a recurring tumour or metastases have a survival rate of less than 20%. It is therefore vital to find predictive methods to determine which children are at greatest risk of metastases or recurrent disease.
For this purpose, CRIS has been supporting Dr Enrique de Álava’s team for years through a project aimed at improving the diagnosis of Ewing sarcoma. The team developed a simple methodology that makes it possible to identify patients with certain chromosome abnormalities that are associated with a worse prognosis. This methodology, which is based on a technique called FISH, often used at hospitals, proved to be just as sensitive and predictive as much more complex and expensive methods. FISH (Fluorescence In Situ Hybridisation) is a technique that uses a small DNA sequence with a fluorescent molecule attached to it. It tends to be used to detect chromosome abnormalities in a sample of patients.
There are also other sarcomas that resemble Ewing sarcoma under the microscope. These are called “Ewing-like sarcomas”. However, unlike Ewing sarcomas, there are no clear guidelines for the diagnosis and classification of Ewing-like sarcomas to allow appropriate personalised therapies for each child. In fact, there are so few publications about these tumours that very little is known about them despite their seriousness.
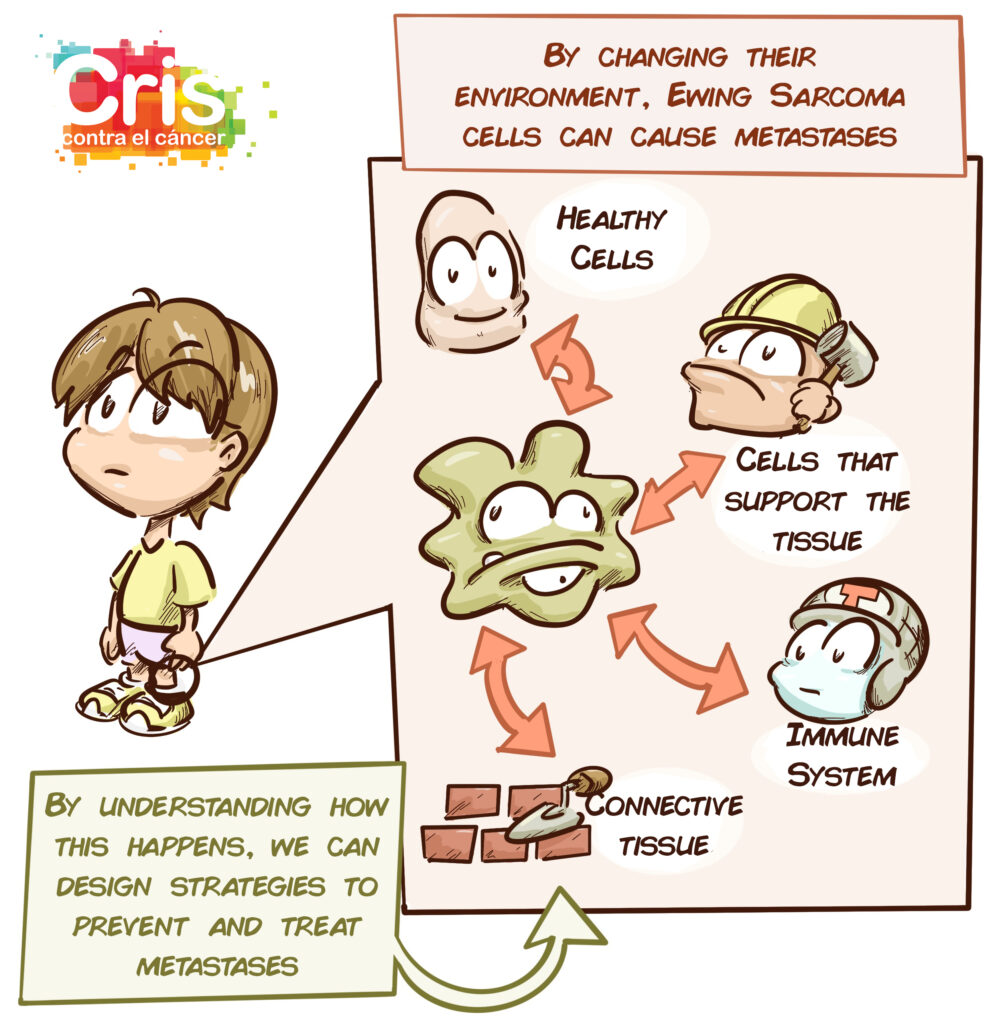
Over the past decade, however, great advances have been made in understanding how these tumours work and how they interact with their environment. The relationship between the tumour cells, surrounding cells and connective tissue network (known as the extracellular matrix) plays an enormous role in tumour growth, aggressiveness and spread. The cells interact with one another, send signals, contract and expand depending on how dense and flexible their surroundings are.
Objective:
The objective of this project is to identify factors in the connective tissue network (extracellular matrix) that allow us to describe, classify and predict the behaviour of Ewing-like sarcomas and Ewing sarcomas. Tumour cells will also be studied genetically to find out which abnormalities may be involved in the modification of their environment and the appearance of metastases.
This study will allow us to identify new ways of determining the characteristics and risk of recurrence and spread of these sarcomas and will also help us to identify new therapeutic targets that may be developed into more effective and personalised treatments.
Recent Progress:
Over the past year, despite the COVID-19 pandemic, the team has managed to identify 27 cases of Ewing-like sarcomas in children. This is a significant number of cases since this is a rare disease that occurs very infrequently. Nevertheless, new cases will continue to be added to the study so this number will increase over the next few months. In fact, this sample is one of the most numerous for this type of tumour.
The investigators are studying the samples collected up until now. The aim is to analyse elements of the connective tissue network, immune system components and tumour cells. The investigators are also analysing damage to DNA using high-resolution techniques that have never been used before in this type of tumour.
An in-depth molecular analysis of these samples, especially their RNA, is also being conducted: If you consider DNA to be a library containing all the instructions for the appearance and behaviour of the cells, RNA would be specific volumes, the instructions that the cell extracts to perform a specific function. Depending on which RNAs the cell is using, we can understand its behaviour and use this knowledge to improve diagnoses or even develop targeted therapies.
For now, preliminary results indicate that higher levels of a protein called vitronectin, which forms part of the cells’ normal environment, could give tumour cells the potential to produce metastases. In the words of Dr Rosa Noguera, it would work like “butter on a sledge”. These results are currently pending publication in scientific journals, something that it expected over the coming months.