Introduction
During the last few years, the conception of how cancer develops has changed radically. Tumours are no longer considered isolated entities that grow in isolation from their environment, and it has been shown that tumour cells maintain a close relationship, communication, and manipulation of their environment.
Among the components of the tumour environment, the immune system plays a key role. Under normal conditions, our immune cells (cell troops) can identify and destroy any tumour cell with very high efficacy. However, sometimes tumours can turn off, confuse, or evade the immune response against these altered cells.
Over the past few years, it has been demonstrated that certain immune system switches can be manipulated to reactivate and/or redirect immune responses against the tumour. This makes it possible not only to eliminate tumours, but also to develop a durable response and prevent future relapses. This therapeutic manipulation of the immune system is called immunotherapy, and its implementation in cancer has revolutionised the way these pathologies are understood and treated.
These types of treatments are more precise and advanced therapies and are showing very promising results. However, different immunotherapy treatments are not yet effective for all patients, so there is an urgent need for their development:
- Methods to predict which patients will respond correctly to current immune therapies.
- New therapeutic approaches and strategies that broaden the range of action of immunological therapies to reach as many patients as possible.
Unit Description:
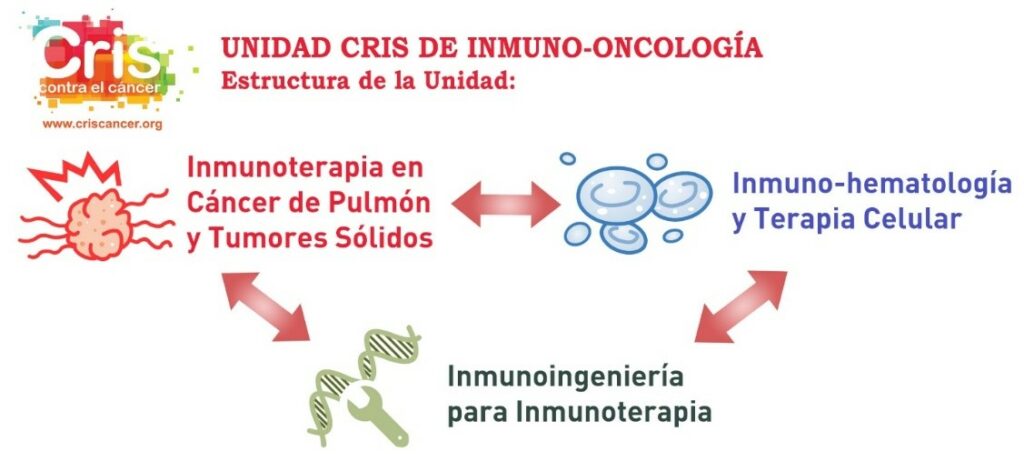
The new CRIS Immuno-Oncology Unit is a pioneer in Spain and approaches immunological therapies from various strategies and disciplines to develop them in the optimal and efficient manner. Three of the most important researchers in Spain in this field are involved. The three branches of the Unit are:
| CRIS IMMUNO-ONCOLOGY UNIT | |
| Immunotherapy in Lung Cancer and Solid Tumours | Immuno-Haematology and Cell Therapy |
| Immunoengineering for Immunotherapy |
- Immunotherapy in Lung Cancer and Solid Tumours: Directed by Dr. Luis Paz Ares, Head of Medical Oncology at the 12 de Octubre Hospital, who also heads the CRIS Immunotherapy Unit.
- Immunohaematology and Cell Therapy: Directed by Dr. Joaquín Martínez, head of Haematology at the 12 de Octubre Hospital and Scientific Director of CRIS.
- Immunoengineering for Immunotherapy: Directed by Dr. Luis Álvarez Vallina, Head of the Cancer Immunotherapy Unit (UNICA) at the 12 de Octubre Hospital and the Immunotherapy and Cell Engineering Laboratory at Aarhus University in Denmark.
These three projects collaborate closely in the development of new and innovative therapies, new prognostic methods and predictive response biomarkers, and their rapid implementation in clinical trials.
Immunotherapy in Lung Cancer and Solid Tumours:
Dr. Luis Paz Ares’s group is divided between the Institute of Biological Research of 12 de Octubre Hospital and the Spanish National Cancer Research Centre (CNIO). Its main objective is to identify characteristics of patients that allow them to predict the response to immunotherapy, the development of personalised therapies and new clinical trials.
On the other hand, they have also initiated a line of research on microcytic (small cell) lung cancer, one of the most fatal lung cancers that lacks adequate treatment.
Immunohaematology and Cell Therapy:
This multidisciplinary group led by Dr. Joaquín Martínez-López develops its work between the Translational Haematology Research Unit at the 12 de Octubre Hospital and the Spanish National Cancer Research Centre (CNIO). The main research focuses of this group are on next-generation cell therapies and the design and development of clinical trials.
Immunoengineering and Immunotherapy (Cancer Immunotherapy Unit, UNICA):
Dr. Luis Álvarez Vallina’s group has vast experience in developing therapies based on genetic engineering. Some of his works were in fact the cornerstones of current CAR therapies. The fundamental participation of Dr. Alvarez-Vallina in this Unit allows the generation, development, and immediate introduction of the most innovative and revolutionary therapies in clinical trials in solid and haematological tumours.
Overall, it is a unique multidisciplinary unit in Spain, which will address the treatment of cancers of all types through various immunological strategies, generates innovative clinical trials and is always at the forefront of new treatments.
Latest developments:
Solid Tumour Group:
New markers of response to immunotherapy in lung cancer
Nearly 30,000 cases of lung cancer are diagnosed each year in Spain, making it the fourth most diagnosed cancer. However, lung cancer causes the most deaths per year, almost 23,000 (data from SEOM). Immunotherapy is one of the most promising therapies for these types of tumours. However, not all patients respond to these therapies.
One of the keys to achieving widespread use of immunotherapy and obtaining its maximum benefits is to know:
- which patients can benefit
- know it in advance and
- make it work in those patients in whom a priori it would not.
With these objectives in mind, the laboratory has obtained tumour material from 200 patients with non-small cell lung cancer, in early stages (i.e., they have not progressed or metastasised). They are undergoing an in-depth analysis in which they are studying, on the one hand, how the immune system behaves in the tumours of these patients. On the other hand, DNA mutations that may be related to the behaviour of each tumour are sought. The goal is to perform a comprehensive immune profiling and establish predictions as to which of these patients might benefit from immunotherapy. After the study of the 200 patients, it has been observed that there are certain mutations (characteristics of certain lung tumour subtypes), which predict the behaviour of the immune system in these patients. The associations found between the peculiar mutations of the tumours and the immune response of these patients help us to predict how they will respond to different immunotherapy strategies. (See illustration)

| Lung cancer patient samples | Samples from patients treated with immunotherapy and have not responded | Samples from patients treated with immunotherapy and have responded |
| Making a profile of each patient’s immune system | Profiling of each patient’s immune system | Profiling of each patient’s immune system |
| Prediction of response | Validation of predictions |
With this data in the hand, it may be possible to predict which patients will respond better or worse to immunotherapy. To validate these predictions, they are being studied in samples from more than 32 patients who received different types of immunotherapy. Some of them responded well, others did not. The idea is to test whether the immunological and genetic profile correctly identifies responding patients. If this is the case, one of the most important milestones in current research will have been achieved: These results will be invaluable from the point of view of patient prediction and follow-up and is a key step in the personalisation of therapies.
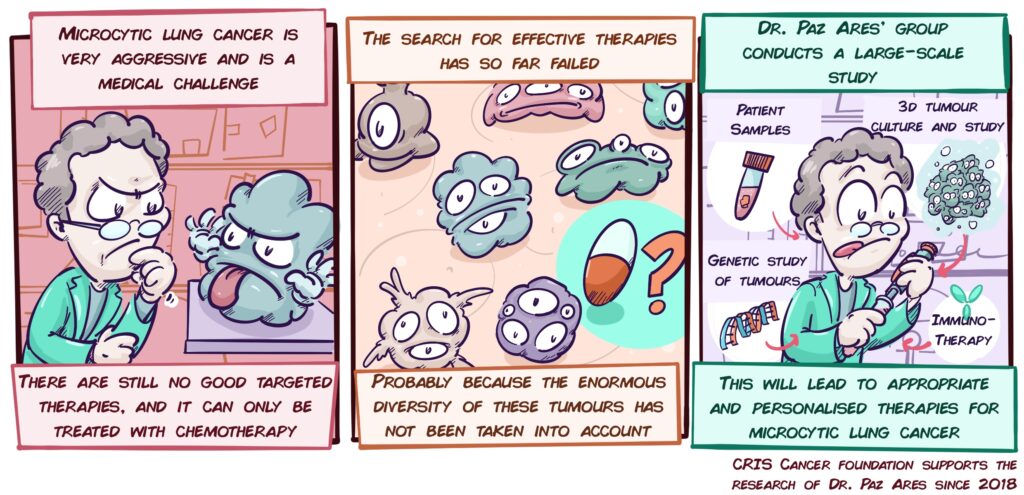
Personalised treatments in small cell lung cancer (microcytic)
Among lung tumours, 10-15% are small cell lung or microcytic tumours (SCLC). These variants are very aggressive and generate metastases very quickly. Although current treatments (platinum-based chemotherapy) work for a while, patients soon stop responding, resulting in a median survival of just over a year.
However, studies to date that look for new therapies have not considered that SCLCs are not all the same, that depending on the patient they may have very different characteristics, and that therefore, not all patients will respond to the same therapies. For example, a tumour may have defects that make it more sensitive to drugs directed against DNA repair, yet it does not respond well to immunotherapy; and vice versa. This may be one of the reasons that have led to not finding effective therapies; we should not look for general therapies, when what works for one group of patients may not be effective for others.
With this in mind, Dr. Paz Ares’ group, a world leader in the study of this type of cancer, has launched an ambitious project that addresses this problem by studying Microcytic Cancer in unprecedented depth. A large number of patient samples, a multitude of laboratory models, and three-dimensional cultures of tumour cells (organoids) will be used to study the diversity of these tumours. The different variants of gene alterations or other molecular alterations will be analysed, but we will also study how different tumour cells interact with their environment, the other cells, and the immune system. In addition, the latest technological advances will make it possible to study cells, their interactions and molecular details with a resolution never seen before.

| Microcytic lung cancer is very aggressive and is a medical challenge | The search for effective therapies has so far failed | Dr. Paz Ares’s group conducts a large-scale study |
| Patient samples 3D tumour culture and study Genetic study of tumours Immunotherapy | ||
| There are still no good targeted therapies, and it can only be treated with chemotherapy | Probably because the enormous diversity of these tumours has not been taken into account | This will lead to appropriate and personalised therapies for microcytic lung cancer |
| Cris cancer foundation has been supporting the research of Dr. Paz Ares since 2018 |
This will make it possible to describe and test effective treatments for different groups of patients, to explore combinations of immunotherapy with other types of treatment, and to validate new strategies (biomarkers) that will make it possible to predict and choose the treatments that will most benefit each patient. This is a fierce bid to find effective solutions for one of the tumours that have the greatest impact on our society.
Immunoengineering and Immunotherapy (Cancer Immunotherapy Unit, UNICA):
Since its creation in 2018, this Unit has grown rapidly, and now has 13 researchers working at full capacity. Much of their efforts are devoted to using the most advanced techniques to generate new antibody-based therapies.
Antibodies are molecules that act as guided missiles, identifying and binding with very high efficiency and specificity to the molecule against which they have been designed. Special antibodies are called Bispecific antibodies. These bind not to one molecule, but to two different molecules. This means that they can be designed to bind and attract tumour cells and T lymphocytes to each other (as we can see in the image), making the immune response against the tumour much more effective.

| Tumour cell | Bispecific antibody | T lymphocyte |
These therapies have some advantages over CAR-T cells, a next-generation therapy. The latter consists of introducing a receptor into a patient’s T lymphocytes, which helps them to identify and attack tumour cells. These therapies are revolutionising the treatment of some leukaemia and lymphomas, but they have some drawbacks, including the fact that only a limited number of CAR-T lymphocytes can be introduced into the patient. Bispecific antibodies are a much simpler and safer therapy and have the ability to act on many T lymphocytes. In addition, they can trigger a more complete immune response than CAR-T lymphocytes. However, they also have important limitations: bispecific antibodies, once injected into the patient, are not very stable and tend not to last very long. With the idea of solving the problems of both CAR-T cells and bispecific antibodies, they are applying an innovative take on the previous concept: Stab cells, the star product of this Unit.

| CAR-T | Bispecific antibodies | STAB cells | |
| Pros | Receptors are introduced into T lymphocytes and inoculated | They bind lymphocytes to tumour cells | T lymphocytes are created that release bispecific antibodies |
| Receptors direct the lymphocyte to tumours | T lymphocytes destroy the tumour cell | Antibodies bind lymphocytes and tumour cells | |
| Cons | T lymphocytes destroy the tumour cell | It’s easier than using CAR-T | They combine the good of the CAR-T and bispecific antibodies |
| A limited number of CAR-T lymphocytes can be introduced | They do not last very long in blood |
It consists of extracting T lymphocytes from the patient and modifying them so that they can produce and release these bispecific antibodies. They are then re-introduced into the body. The advantage of this type of therapy is that it is not necessary to inject antibodies into the patient, but are they are produced by the patient’s own troops. Therefore, these therapies have the best of both bispecific antibodies, and CAR-T cells, which last longer in the patient.
The results of the Stab cells are excellent. In laboratory models (both in vitro and in vivo) they can produce the same degree of tumour cell killing as CAR-T cells. Not only that, but fewer STAb cells than CAR-T cells are needed to produce the same effect, which will allow working with lower and safer cell doses in patients. The results in animal models have been so positive that the group is preparing a clinical trial for patients with leukaemia and lymphoma using this therapy, which is likely to open over the next year.
A key feature of Stab therapies is that they can be applied to many other types of tumours. For example, by changing the antibody released by T lymphocytes, this therapy can be directed against other tumours, such as multiple myeloma (using an antibody that binds to a very common molecule in myeloma, BCMA). The team is currently conducting preliminary studies which, if positive, could also lead to the development of a clinical trial for multiple myeloma patients.
The rapid advancement of science requires that different research groups collaborate with each other to develop multidisciplinary projects with a broader scope. Dr. Vallina’s team is aware of this and collaborates with the most important immunotherapy groups in Spain and the world. For example, they are working with groups from the University of Navarra and the Spanish National Centre for Cardiovascular Research (CNIC) to develop new treatments that act on other types of cells, called dendritic cells. These cells are the ones that activate T lymphocytes, so treatments on them could be even more efficient than therapies that focus on T lymphocytes.
One of the most notable collaborations of this group is with the Weizmann Institute of Sciences. Together they have developed a CAR-T cell-based therapy that attacks the abnormal blood vessels that form in tumours. Results in animal models have shown that these CARs would be very efficient in aggressive tumours such as glioblastomas or lymphomas. For this reason, they are preparing a joint clinical trial in which Spanish patients will also be included.
Finally, in another collaboration, this time internal, with Dr. Paz-Ares, they are developing a new project to improve a type of cell therapy that so far has only worked in some types of melanoma and kidney tumours. This therapy is usually based on removing T lymphocytes from the patient’s tumour, stimulating them, and reintroducing them into the patient (the therapy is called TIL (tumour infiltrating Lymphocytes). The problem that both directors of the Immunotherapy Unit are trying to address is that many tumours can inactivate again the lymphocytes that are reintroduced. Thanks to immunoengineering, they hope to solve this problem.