Context
Haematological, or blood cell tumours represent largely incurable cancers today. Blood cancer is the fifth most common cancer in the UK, with over 40,000 people being diagnosed with it every year. There are about 250,000 people living with blood cancer in the UK. One in every 16 men and one in every 22 women will develop it at some point in their lives. More information about these tumours here.
These tumours can be largely controlled, but in many cases, relapse occurs in a fairly unpredictable manner. This means that the survival rate for these pathologies is still around 50%. Research in this field is therefore absolutely essential.
Project Description:
The CRIS Haematological Tumours Unit is a multidisciplinary department that combines clinical research (with patients) and laboratory research within the H12O Hospital Haematological Tumour Care Department. The department provides treatment to hospitalised patients, outpatient care, and is also conducting clinical trials. The operation of this unit allows research results to reach patients quickly through clinical trials to treat these tumours, which are largely incurable today.
The HUNET-CRIS Unit has been accredited by the Community of Madrid for Phase I trials. Currently, 132 clinical trials are being conducted in various haematological diseases and cancers, mainly lymphoma, leukaemia and multiple myeloma. Since its opening in 2013, more than 660 patients have been treated in this unit, in over 250 clinical trials. The innovative treatments used have been shown to be safe, as the percentage of toxicities derived from them has been very low, and there has been no deaths as a result of the treatments.
Significant developments from the start:
The CRIS Unit is a fantastic example of how strong support for research has a direct impact on patients’ quality of life. Since the beginning of its activities in 2013, the Unit has developed at least 15 different treatments, based on the latest technologies. Examples include the following:
Monoclonal antibodies:
Monoclonal antibodies are one of the greatest biotechnology breakthroughs in history. They are like guided therapies, which seek a specific target (usually in the tumour or some cell in its surroundings), sticking to it, and producing a whole series of effects. They can cause the tumour cell to die. They prevent it from feeding and growing and it eventually dies (for example, those that target important tumour molecules, such as Slam7, CD38, BCL2 or CD79), that it stops manipulating its environment and the immune system (Such as those directed against PD-L1), etc. The Unit has implemented several of these treatments, some of which improve survival in more than 30% of patients, and extend the time to relapse by up to 50%.
Bispecific Antibodies:
This is a new take on monoclonal antibodies. They are genetically engineered antibodies that bind to the tumour cell and to an immune system cell (T lymphocytes) simultaneously. This makes it easy for the T lymphocyte to detect and destroy the tumour cell. They are administered as injections, and in some cases can have an effect as potent as that of CAR-T cells. One of them, blinatumomab, has been used to treat different types of leukaemia and lymphoma.
Immunomodulators (or IMiDs):
These are drugs that are usually taken as pills that have a double effect: on the one hand, they eliminate the myeloma cells that are appearing, and on the other, they promote an immune response against the tumour. They are easy to produce and manage. In the Unit they have worked successfully, for example, with a drug called pomalidomide. The fact that they can be used in tablet form is a huge advantage in terms of patient quality of life.
Cell Therapies:
Dr. Joaquín Martínez has specialised in cell therapies throughout his training. With its initiative, the Unit has been a pioneer in Spain in using CAR-T cells for the treatment of lymphomas, leukaemia, and myeloma (Learn more here). These are T lymphocytes to which a detector is added to help them search for, identify and eliminate tumour cells (see illustration). Depending on the type of tumour, they can be up to 80% effective. They are also pioneers in the use of Natural Killer cell therapies (cells specialised in detecting and destroying damaged or altered cells) in blood diseases.
Targeted therapies:
If instead of a generic treatment, patients are provided with a treatment that specifically targets the weak points of their tumour we have many more options to control or cure their disease. This is the basis of targeted therapies, highly personalised treatments, which in many cases, such as ibrutinib, can be administered as pills, and are contributing to the cure of some acute leukaemia.
Latest developments:
Cell Therapies:
One of the main objectives pursued by the Unit is to improve current cell therapies. One of its focuses is on the Natural Killer cells, which have become increasingly prominent in cancer treatment over the last few years, as an alternative to CAR-T. These therapies have revolutionised the treatment of blood diseases. However, in some cases they produce hyperinflammatory and/or neurotoxic reactions that can be dangerous for the patient. In addition, CAR-T cells from a given donor can attack the healthy tissues of the patient receiving the therapy, so the treatment can only be performed with cells from that same patient, which, apart from not always being in optimal conditions, require a very expensive and personalised production process.
By comparison, Natural Killer cells are much safer, and do not cause any of these possible side effects. This makes them an interesting alternative, but they have a number of problems: without manipulation, they are not as effective as the CAR-T lymphocytes, the tumours can also adapt to them, and when they are introduced into the patient, they do not usually last long in the blood stream. To address these problems, and convert Natural Killer cells into solid therapies, the CRIS Unit laboratories, with Dr. Antonio Valeri leading this project, have developed an ambitious research strategy.
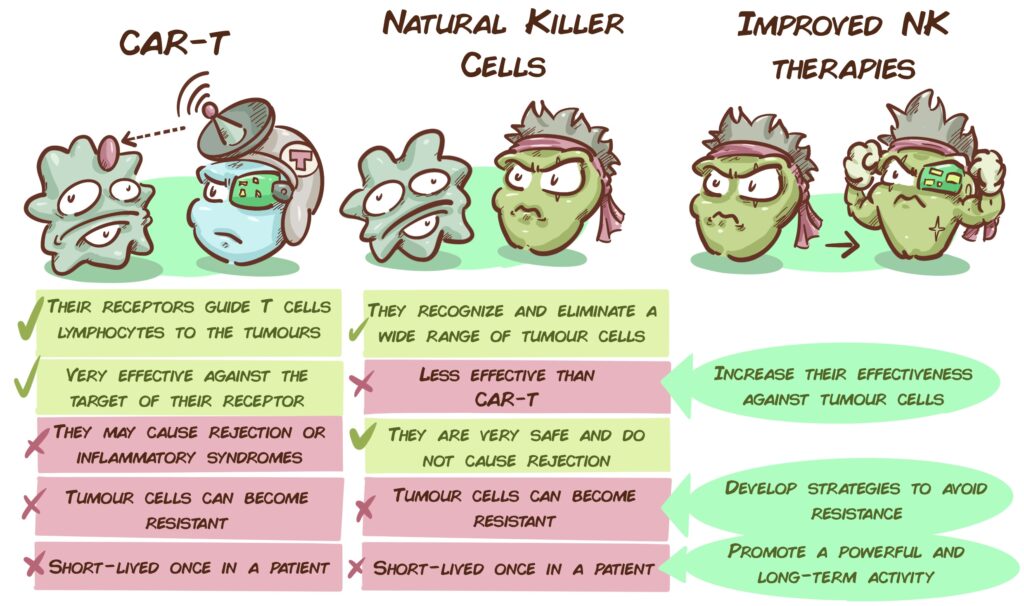
To enhance their effectiveness, work is being carried out to develop therapies with modified NK cells that incorporate an additional receptor, i.e., CAR-NK cells. The receptors/radars added to them include NKG2D (which recognises altered, infected, and tumour cells) and a receptor that identifies BCMA (a molecule that frequently appears in myeloma cells). These models are working very well in vitro against leukaemia and myeloma cells.
However, the introduction of these CAR receptors into NK cells is traditionally very complicated, and it is difficult to obtain enough CAR receptor-modified cells to be an effective therapy. To overcome this obstacle, the Unit’s team has developed a method that allows the NKs not to reject these added receptors and, therefore, makes it easier to obtain a greater number of CAR-NKs.
To combat tumour cell resistance, they have established a novel strategy. As myeloma advances and the immune system tries to fight it, a molecule (HLAE) in the tumour cells starts to appear that switches off the activity of the Natural Killer cells. This means that tumour cells are free to grow, without worrying about NK cells. However, the laboratory has been able to verify that if its natural NKG2A receptor is blocked by neutralising antibodies, NK CAR cells can be re-activated. This finding could be tremendously important, allowing NK cell therapies to work better and stopping tumour cells from adapting.
To increase the duration of NK cells in the patient, the CRIS team has developed a proprietary method to boost the power of NK cells: they last much longer, and unlike normal NK cells, they are much more efficient the second time they face the same threat. As a result of this latter feature, this type of NK cells is also known as, receptor-induced memory-like NK cells, and can be key in the fight against relapses.
Finally, another problem with any cell therapy with CAR T lymphocytes is the cost and effort it requires. They have to be produced from the cells of each patient (to avoid rejection), and that makes the whole procedure much more expensive. In addition, sometimes not enough healthy Natural Killer cells can be obtained from all patients. Ideally, we would like to have a repertoire of cell therapies that are suitable for any patient, that do not cause rejection but are efficient at the same time. Currently, the team is developing a therapy with a cell line of NK cells, in which CARs such as NKG2D and BCMA (mentioned previously) can be introduced, which are low cost and universal for any patient. It is not an easy challenge, but this Unit has managed to overcome the most insurmountable barriers.
Animal platforms to study Multiple Myeloma:
In any case, once a therapy is developed, before it reaches the patients it must be tested in animal models. It is important to have models that reproduce human disease as faithfully as possible, so that the treatments tested give results that can be extrapolated to reality. Until recently, however, there were no good models of multiple myeloma that would allow this disease to be studied correctly. To solve this, the CRIS Unit has developed an absolutely pioneering predictive mouse model. Human multiple myeloma develops in the patient’s own immune environment. Because the researchers are dealing with the human disease, not the animal equivalent, this model is ideal for evaluating treatments that will one day reach patients.
Role of Mitochondria in Multiple Myeloma:
The CRIS Unit not only works on cell therapies. Over the past few years, they have focused on cell energy centres, mitochondria. They observed that as the myeloma advances and progresses, the number, size, and activity of the mitochondria increase. This makes mitochondria a potential therapeutic target, as there are drugs, such as tigecycline, which can be targeted against them. For the moment, they have shown that this is so in cell models, and that in combination with one of the standard therapies, bortezomib, attacking mitochondria can be very effective in slowing the progression of myeloma. This finding has been published in the international journal Cancers.
There is a new take on this advance: If the number and size of mitochondria correlates with whether abnormal cells can progress to more advanced stages of myeloma, we could measure the size and number of mitochondria to predict whether patients will worsen. There is a quick and inexpensive method already implemented in hospitals to do this. Therefore, thanks to this project we could be facing a new and effective predictive test of whether patients are going to get worse.
New targets in the treatment of Multiple Myeloma:
Dr. Miguel Gallardo, who is part of the Unit’s team, has found that a protein called HNRNPK could play an important role in the development of blood tumours such as myeloma. It is a striking protein that under normal conditions helps the cell to overcome periods of stress. However, if it is out of control, it could contribute to healthy cells transforming into tumour cells. This protein is therefore of great interest and the team is analysing in depth how this protein works and trying to determine how to translate this to the clinic.