Context:
Pancreatic ductal adenocarcinoma accounts for 90% of malignant pancreatic tumours. It poses a massive medical challenge for a variety of reasons. Firstly, it is one of the most aggressive and fastest spreading tumours from which a staggering 95% of patients do not survive. Secondly, it is generally diagnosed late and, as there are also no effective treatments for it, mortality rates are extremely high.
Pancreatic cancer is the 10th most common cancer with 10,449 people diagnosed with pancreatic cancer in the UK in 2018. It is the 5th biggest cancer killer in the UK with 9,000 deaths every year.
Although cancer treatments have evolved massively in recent years, frequently used pancreatic cancer treatments continue to be based on therapies with low-specificity and considerable side-effects.
Description of the Project:
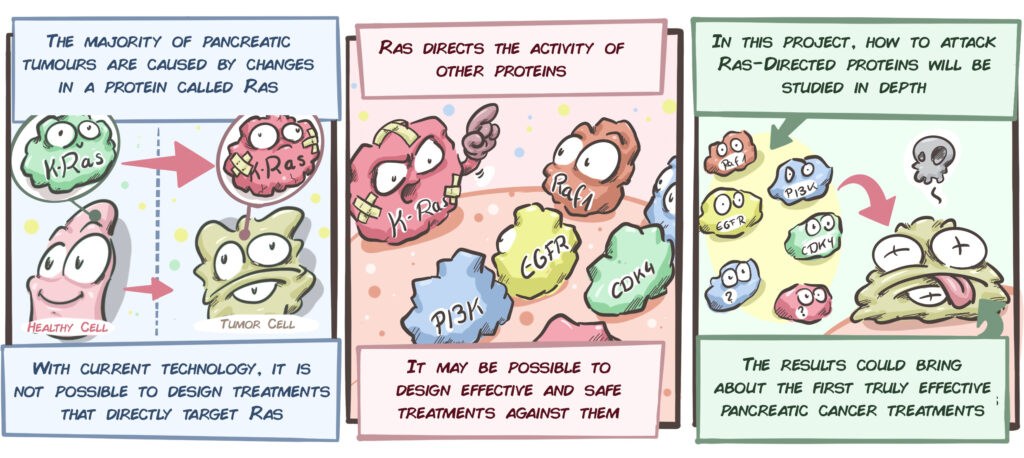
It has long been known that most pancreatic tumours start with mutations in the K-Ras protein, which controls many cellular processes. This is the first step in a process that is followed by the accumulation of other mutations that cause the pancreatic cells to develop into tumours. We might, therefore, believe that treatment targeting K-Ras would help halt the progress of pancreatic cancer. The problem is that, at least at present, it is not possible to attack Ras using drugs. Nonetheless, Ras directs the function of other proteins, so a feasible strategy would be to attempt to block these other proteins to prevent the development of tumours.
This is not as simple as it sounds. This hypothesis means treatments need to be developed that attack proteins and that:
- are directed and regulated by Ras
- if eliminated or separated, the tumours stop growing
- if eliminated or separated, healthy cells survive
After years of concentrated work, Dr Barbacid’s group has found two proteins that meet these criteria: Raf1 and EGFR. The group was able to verify in laboratory models that if these proteins are eliminated simultaneously, half of the pancreatic tumours stop thriving.
These fantastic results are very encouraging as they suggest that treatments against EGFR and Raf1 could be effective for pancreatic cancer patients. However, simultaneously, it opens up a whole host of questions. Firstly, a protein may be important by its mere presence (since it can support other cellular elements) or due to its activity (by modifying other cellular elements). Are tumours without Raf1 and EGFR reduced because these proteins are not there? Why are they not active? It’s a subtle but significant difference: Turning off the activity of a protein is relatively manageable but destroying it with drugs is more complicated (albeit possible).
Another question that arises is whether there are other Ras-related proteins that could be targeted with treatments. Lastly, we have seen that removing EGFR and Raf1 in laboratory models is effective against half of the tumours. What about the other half? Which proteins could be targeted by drugs?
All of these questions are what the ambitious project supported by CRIS is seeking to answer: “Identification and Validation of New Therapeutic Targets” by Dr Barbacid.
Objectives of the project:
- In laboratory models, to check what is causing the tumours to disappear when EGFR and Raf1 are removed: Complete removal? Or just the absence of their activity?
- In the event that tumours only need the activity of these proteins, research will be undertaken to develop suitable drugs.
- In the event that tumours require the complete presence of the protein, research will be undertaken on therapeutic strategies to specifically destroy these proteins in patient tumours.
- In parallel, a similar study will be carried out on other proteins that are controlled by Ras. Emphasis will be put on PI3K and CDK4 proteins.
- Lastly, tumours that grow even in the absence of EGFR and RAF1 will be screened for new targets that can be treated.
To conclude, this is a large-scale project, which has significant potential to develop new therapies for what is, currently, a devastating cancer.