Investigator in charge of the project: Dr.Antonio Pérez Martínez
Background:
Childhood cancer is rare – around 1,900 new cases are diagnosed every year in the UK (in children aged 0-14 years). This means that around one child in 500 will develop some form of cancer by the age of 14 years. Over the last decade in the UK (between 2004-2006 and 2014-2016), AS incidence rates for cancers in children (girls and boys combined) increased by 8%. In girls AS incidence rates increased by 9% and in boys rates remained stable
The highest incidence rates for all children’s cancers combined are in the under-fives for both sexes, with almost half (46%) of all cases in children being diagnosed in this age group (UK, 2014-2016).
Despite the shocking nature of the situation, treatments for childhood cancer have stagnated for the last 30 years, as childhood cancer is very different to cancer in adults. This is because we do not understand the mechanisms that cause tumours in children or the reasons why their immune system is unable to combat them
This makes it imperative to perform clinical research to be able to understand how the cancer occurs, to try to prevent it and to combat it using more effective therapies with reduced side effects. This comes through research and conducting clinical trials. These trials constitute the ideal scientific setting, where patient and healthcare professional safety is preserved, and proof-of-concepts achieved in the laboratory are explored. In our country, we still need to drive and conduct clinical trials in children to help advance therapies against childhood cancer.
Project Description:
Coordinated by Antonio Pérez Martínez, the CRIS Unit of Advanced Therapies aims to move the treatment of childhood cancers forward and to create a multidisciplinary, integrated team to combine cutting-edge research, clinical trials and the most advanced therapies. Doctors, researchers, nursing, geneticists, immunologists, bioinformaticians, quality and data managers, etc. will come together to treat the most difficult cases of childhood cancer in increasingly personalised ways.
Its objective is both to improve current therapies for childhood cancers and to develop new ones and to ensure their availability to the patient (translation) in the most agile way possible. This CRIS Unit will enable on-site research, the development of innovative therapies through clinical trials, and an increasingly personalised and unique clinical practice for each patient.
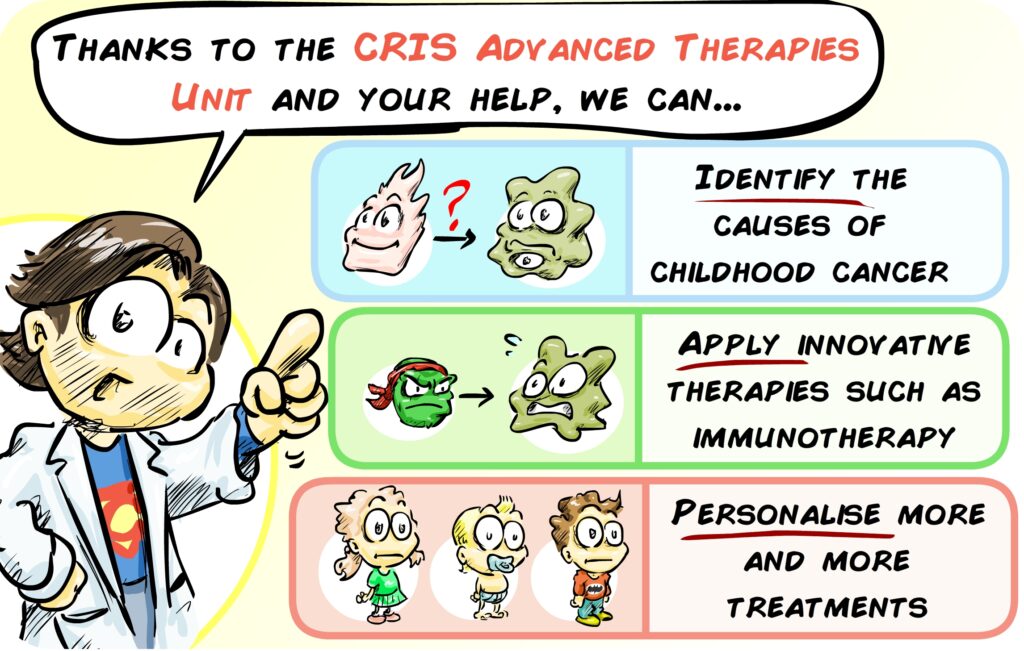
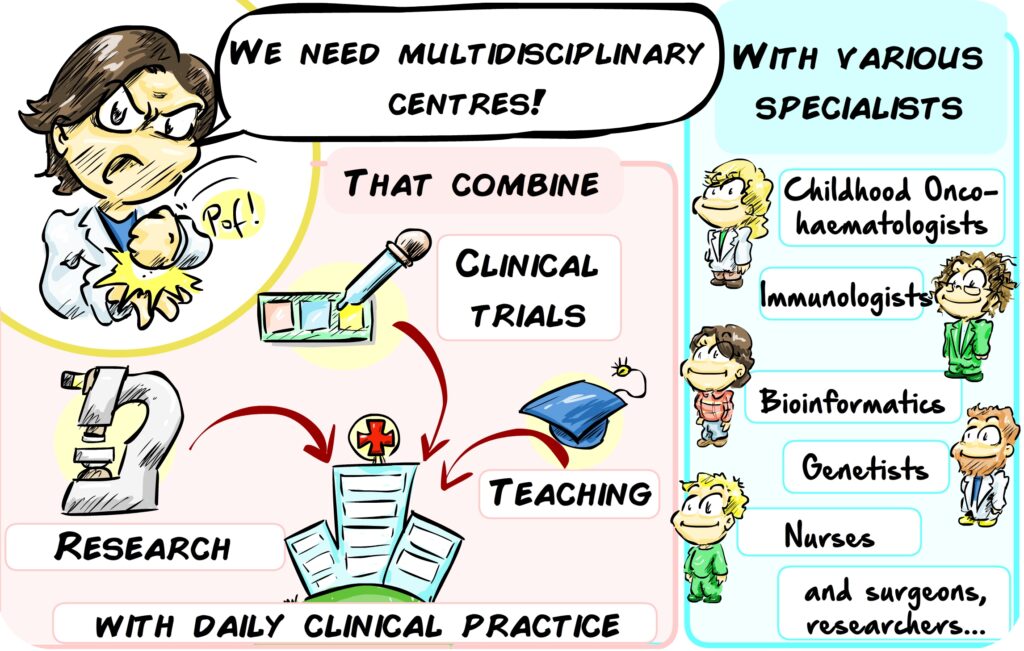
Structure of the CRIS Advanced Therapies Unit:
The Advanced Therapies Unit is an interdisciplinary working structure, made up of professionals from different fields. The CRIS Cancer Foundation has financed the construction of the Unit’s physical location on the 8th floor of the Maternity-Children’s Hospital at the La Paz University in Madrid. The cost of construction of this Unit was €1,000,000, and consists of:
- 10 rooms.
- 4 state-of-the-art isolation rooms for haematopoietic progenitor transplants.
- Clinical trials area.
- Treatment preparation area.
- Specialised laboratory
- Work area for researchers, clinicians and clinical trial monitors.
The Advanced Therapies Unit’s true strength lies in the coordinated work of professionals from multiple disciplines, who work together to accelerate the transformation of new scientific knowledge into innovative treatments for children who have no other therapeutic options. The CRIS Cancer Foundation funds these people, the true structure of the Advanced Therapies Unit, with a contribution of €1,000,000 over three years
Professionals funded by CRIS:
- Onco-Haematologist Paediatrician.
- Geneticist.
- Bioinformatician.
- Molecular Genetics Laboratory Technician.
- Cell Therapy Laboratory Technician.
- Project and Clinical Trials Manager.
- Expert in regulatory aspects of advanced therapy medicinal products.
- Medical Writer.
- Pre- and Post-doctoral Researchers.
One of the distinguishing features of the treatments being developed in the Advanced Therapies Unit are the next generation cell therapies. These supply the patient with strengthened immune system troops, specialised in destroying tumours.
However, these therapies require very precise, delicate cell manipulation, which needs to be carried out under extremely high standards of quality in settings that combine manual manipulation with sophisticated automation.
Some recent Unit results:
The Unit was launched in October 2018, and has been operating at full capacity since the beginning. Since its opening, more than 578 children with a wide variety of pathologies have been treated: various types of diseases and blood tumours, such as acute lymphoblastic leukaemia, acute myeloblastic leukaemia, mixed phenotype leukaemia, aplasia, primary immunodeficiencies or Hodgkin’s lymphoma. A large number of solid tumours have also been treated including Ewing’s Sarcoma, Osteosarcoma, Medulloblastoma, Neuroblastoma, Gliomas Cellular therapies and immunotherapy are routinely applied in this CRIS Unit, including CAR-T cells, various targeted therapies against characteristic mutations of individual tumours of certain patients, and immunotherapy.
Over the last few years, the group directed by Antonio Pérez Martínez has received several awards and recognitions, among which we highlight:
- Group of Excellence: The Children’s Haemato-Oncology Department at La Paz Hospital has earned the Best In Class (BIC) 2020 as the Best Reference Unit in Advanced Cell Therapies.
- The COVID-19 project has been recognised as the Best National Project, of the 2019 Excellence Prizes Award by IdiPAZ.
- The COVID-19 Project (COVID-19 therapy lymphocyte library formation) has been selected to participate in the Healthstart madri+d 2020 edition.
- The COVID-19 project has been selected as one of the 20 finalists among more than 700 projects in the virtual Hackathon “VenceAlVirus” 2020
- Best scientific publication awarded by the scientific societies GETH and SEHOP. 2017
Regarding the projects and trials being carried out in the Unit:
Gabi Project:
Our body has an efficient army specialised in rejecting tumours. There are several types of cells that work together in our defences against cancer, but in short, we could talk about two:
- Natural Killer cells, which patrol the body and eliminate any cells in poor condition or suspected of being tumours, and T lymphocytes, elite cells of the immune system. Most NK cells have a surface receptor (NKG2D) that identifies a molecule that appears on the surface of the cells when they are in poor condition, including when they become tumours. When this receptor identifies a cell in poor condition, the NK eliminates it.
- T lymphocytes, elite cells of the immune system. There are millions different ones, and each specialises in one type of threat or enemy. They are very effective but very selective, unlike NKs which attack a greater number of threats, but less effectively than T cells.
The Gabi project will try to unify the best of NK cells and T lymphocytes thanks to CAR technology:
Memory T cells will be extracted from a donor and a special receptor (CAR) will be constructed that adds the NKG2D detector typical of NK cells to the memory T cells. In this way we will have memory T cells that:
- As good memory T cells, they will be long-lasting and highly effective and be capable of destroying tumours.
- In addition, they will have the ability to recognise a broad spectrum of tumour cells, as NK cells do.
Essentially it consists of making an NK-T cell fusion with the best of each. The introduction of these cells will mean reinforcements that will remain in the body for a long time, will be very effective against tumours and will also recognise a huge spectrum of tumour cells.
This trial requires a large number of validations and authorisations, due to the complexity of the GAby-Cells generation process. This type of cell has already been administered in some cases as compassionate use, but the use in clinical trials requires a long process, which is coming to an end:
The production of CAR-NKG2D cells must be carried out in a highly clean, isolated and safe room, called a Clean Room. This Clean Room is already built and enabled, but requires a series of validations and authorisations in order to become operational. These administrative procedures have suffered several delays due to the COVID-19 pandemic, but despite these delays, it is expected that patients will be enrolled in this innovative trial by Autumn 2021.
Fast-Track Project:
Childhood cancer is a group of diseases that appear as a consequence of a series of genetic alterations (called mutations) that cause the transformation of a healthy cell into a tumour cell. It is essential to identify the exact alterations present in the tumour cells of each patient in order to reach a specific diagnosis and find the most effective treatment. Conventional techniques are available to identify some of the most common genetic alterations. However, sometimes children present more than one of these disorders or other less known disorders that are not detected by these tests. Massive sequencing is a system for analysing genetic material that has the potential to detect an enormous number of mutations in a single test.
The Fast-Track project consists of applying these massive sequencing techniques to childhood cancer patients. In this way, an exhaustive characterisation of the profile of each patient’s alterations will be made. This allows us to:
- Refine the patient’s diagnosis and more accurately determine the type of tumour for each child.
- Better identify tumour cells, design tools to better track the progression of each patient, and react more quickly in case of relapses or disease progression.
- Rapidly design new treatment strategies and search for drugs that specifically target the alterations in that patient’s tumour. This will be especially important in those children who do not respond to conventional treatments or are relapsing.
The procedure is designed so that, if necessary, genetic data and possible alternative treatments can be obtained in less than two weeks. This speed of analysis can be key, especially in cases of relapses.
During the last year, tumours have been sequenced in 36 patients, of whom 9 were relapsing or refractory (resistant) to treatments. Of the latter, 3 had mutations that could be targeted by existing drugs. As a result, they have been able to receive personalised treatment specifically targeted against the weak points of their disease.
There are numerous examples of the importance of this personalised study of genetic alterations in the tumours of children with cancer. For example, the CRIS Unit successfully treated the fibrosarcoma of a new-born girl after analysing the genome of the tumour and identifying a genetic mutation that causes other diseases in adults (a mutation in the TRK gene). Fortunately, there is a drug against this mutation, larotrectinib, which made it possible to cure the girl and to do so with an excellent quality of life.
On another occasion, a girl with acute myeloid leukaemia, which is rare in children, did not respond to any treatment. Advanced genetic characterisation allowed them to identify that this patient’s tumour had a mutation in a gene called FLT3. Thanks to this finding, it was possible to seek a treatment directed against FLT3, and it was possible to stop the disease and finally, after a bone marrow transplant, the girl recovered.
In short, this case-by-case analysis strategy can save a large number of lives.
The Hercanin Project:
At the end of 2018, a Paediatric Cancer Predisposition Unit was created, a multidisciplinary group aimed at identifying mutations that increase the risk of developing a tumour, both in children and in other family members.
Genetic analysis of the child patients revealed alterations that could cause tumours in other members of their families. Of a total of 50 families studied, 40% of them were found to have some alteration that could increase the risk of developing certain types of cancer.
On the other hand, samples from more than 270 children with cancer have been studied, in which possible alterations in more than 60 genes have been analysed. In several of these patients, certain mutations have been detected that may indicate a certain predisposition to develop these pathologies. The results obtained in these analyses are also being used to conduct in-depth scientific studies on cancer predisposition in childhood patients, a field about which little is known.
Along these lines, the team has recently published a study in the prestigious journal Clinical and Translational Oncology, analysing the predisposition to cancer of late foetal stages and neonates.
In any case, the results of these studies are extremely important, since they make it possible to anticipate the appearance of other cases of childhood cancer in relatives of patients in whom these mutations have been found. This allows the Paediatric Cancer Predisposition Unit and the medical Oncology Department at La Paz University Hospital to provide genetic counselling and appropriate follow-up to these families.
Neuroblastoma Project:
Neuroblastoma is a type of tumour that mainly affects children under five years of age, and represents almost 9% of all cases of childhood cancer in Spain. It originates from immature nerve cells, neuroblasts, cells that originate neurones and nerve fibres. Normally these cells are present in the foetus, when needed, and after birth they disappear or mature into neurones. However, in some children these neuroblasts do not disappear, and as they have a great capacity to divide, they can produce tumours.
Numerous advances have been made in the treatment of neuroblastoma, but in the face of metastases, the survival rate is still less than 30%. It is therefore essential to identify the mechanisms of metastasis in order to combat them effectively.
Dr. Antonio Pérez’s team has been able to prove that Natural Killer cells could be effective in eliminating neuroblastoma cells. As previously explained in the GABI Project, NK cells usually recognise abnormal cells through a receptor called NKG2D. To achieve a potent, long-lasting and effective therapy against neuroblastoma cells, Dr. Antonio Perez’s team has introduced NKG2D into T lymphocytes, creating a mixed NK-Lymphocyte T cell (GABI cells).
However, neuroblastoma cells are diverse, and tumours from several patients sometimes hide the targets that allow NKG2D to identify them as abnormal. When this happens, neither the NK nor the GABI cells are able to identify and destroy them. That’s why the lab is currently working on finding ways to stop tumour cells from hiding these targets.
Therefore, the aim of this work is to make neuroblastoma tumour cells visible again to NKG2D. At that time, we will be able to apply cell therapies with NKG2D T lymphocytes (GABI cells) and effectively destroy the tumour.
The team is currently working on laboratory models. If the results are positive, they hope to be able to propose a clinical trial with this therapy in about a year.
Children’s Sarcomas Project:
The design of this project is very similar to that of the Gabi trial (see above). CAR-NKG2D cells will be used to fight tumour cells from paediatric patients with sarcoma.
Authorisation has been received from the Spanish Agency of Medicines to conduct a clinical trial. As in the case of the Gabi project, the validation and authorisation of the Clean Room to produce the CAR-NKG2D cells is required to perform this trial, so the trial will start when the use of this room is authorised and validated.
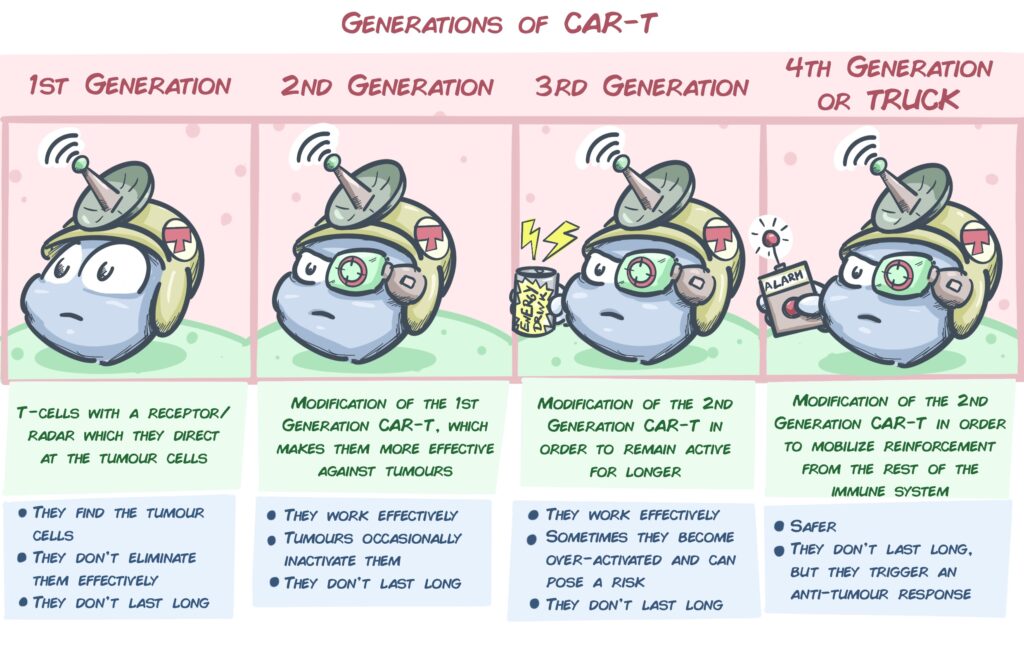
In addition, work is also being done on a new type of CAR called TRUCK, with a great future in solid tumours such as sarcomas. These are T lymphocytes that not only have a receptor added to attack the tumour, but are also equipped with a system to recruit other cells of the immune system against the tumour.
Brain Cancer (Medulloblastoma) Project:
Medulloblastoma is the most common malignant brain tumour in children. Despite modern treatment techniques, 30-40% of children may have relapses or metastases, with few effective treatments. Among the most promising treatments for this type of tumour are cell therapies based on immune system cells.
As in several of the previous projects and studies, this project, divided into 2 parts, is based on T lymphocytes and Natural Killer cells.
- On the one hand, thanks to the excellent results obtained in the laboratory in medulloblastoma models using Natural Killer cells, a Phase I clinical trial will be launched in which NK cells will be infused in relapsed childhood medulloblastoma patients. These cells have already shown great effectiveness in the laboratory in the identification and destruction of brain tumour cells, and positive results are expected.
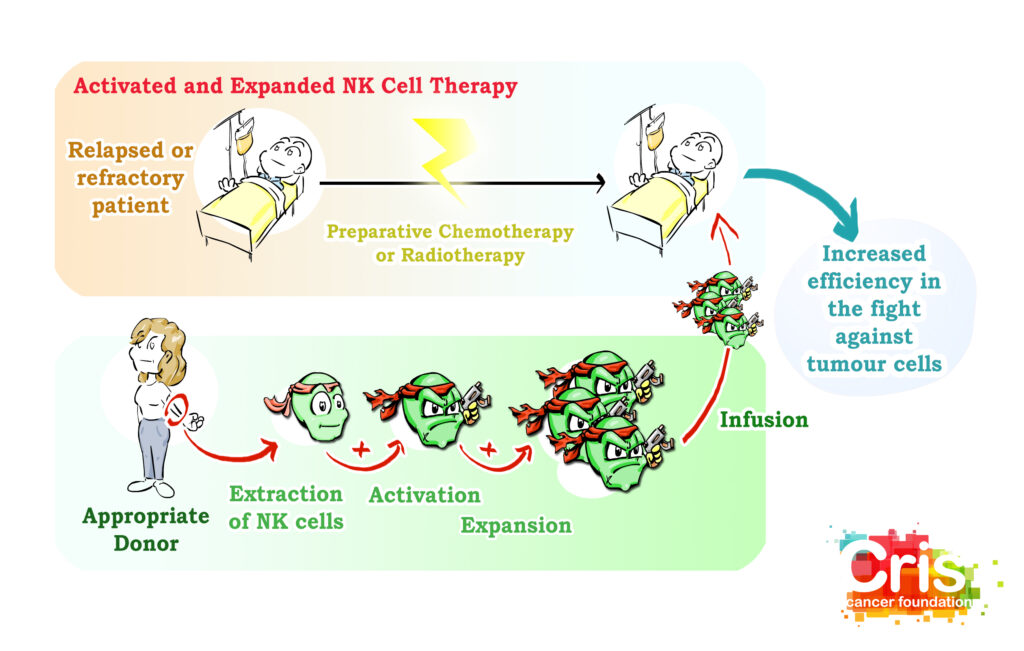
2) On the other hand, this project continues to improve Natural Killer cells to fight against medulloblastoma. To this end, the safety and efficacy of an innovative therapy based on CAR cell technology is being tested in the laboratory in order to lay the groundwork for a future clinical trial for patients with medulloblastoma. Several types of CARs are being worked with:
- CAR with the NKG2D receptor to T lymphocytes, as in the Gabi and Childhood Sarcoma projects. It identifies most abnormal cells and destroys them.
- CAR with CD16 receptor. This receptor makes it possible to label tumour cells with an antibody and cause CAR cells to pounce on them.
- DUAL CAR NKG2D-CD16. In this case a novel double CAR is added to the lymphocytes, which identifies any abnormal cells and would also attack the cells that we label with antibodies.
Intensive work is currently under way in the preparation of the Phase I clinical trial. This will be launched as soon as the facilities and cell therapy manufacturing process at La Paz University Hospital are authorised and the Spanish Medicines Agency authorises the start of the trial.
Cellular Therapies in the Treatment of COVID-19:
In an extraordinary way and due to the severity of the situation, the CRIS Unit has launched a clinical trial that adapts the cell therapies developed for childhood cancer to patients with severe COVID-19 (Learn more here).
Invest in life. Become a regular donor.
Our life depends on research; it is thanks to research that will be able to win the battle against cancer. We will only achieve this with people like you.
Investing in research is investing in life.