Background:
Brain tumours are the most common tumours that develop in children. Children of any age may be affected. About 400 children in the UK develop brain tumours each year. This includes medulloblastoma, which usually starts in the region of the brain known as the cerebellum. It is one of the most common tumours and, unfortunately, one of the most aggressive. Despite advances in recent years, over 40% of children with medulloblastoma experience recurrences, which are often associated with a low cure rate.
Significant improvements have been made in the treatment of children with refractory medulloblastoma, largely through very specific chemotherapy regimens. However, it is essential for more targeted and novel therapies to be identified to enable this group of young patients to receive effective treatment.
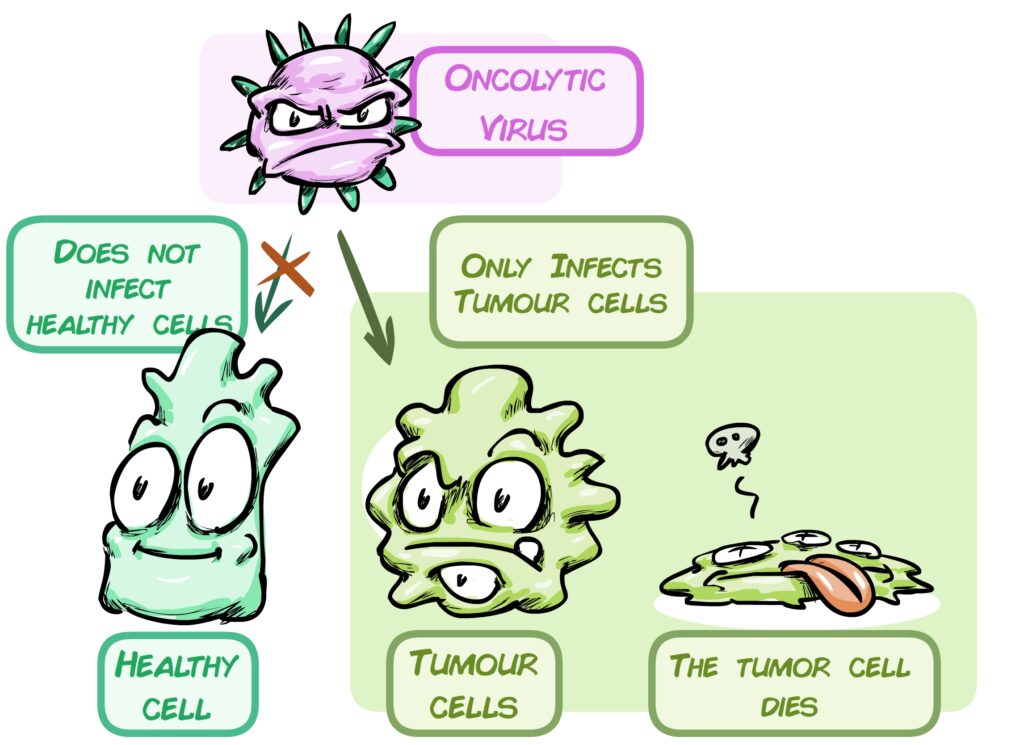
Over the past 14 years, Dr Álvaro Lassaletta’s team has been working on oncolytic virus-based immunotherapy: this virus ignores healthy cells and specifically infects and kills tumour cells. CELYVIR, the treatment administered to this group involves these viruses being carried by mesenchymal cells, which are injected into the patient and the virus is released into the tumour. Its safety has been demonstrated in a variety of childhood tumours, such as neuroblastoma and sarcomas. We now aim to check its safety and efficacy on brain tumours.
Description of the Project:
The project consists of a Phase 1 clinical trial involving patients aged 0-22 years old who have been diagnosed with metastatic or refractory medulloblastoma.
The objective is to evaluate the safety of treating patients with these oncolytic viruses in paediatric, adolescent and young adult patients. The project also seeks to evaluate the antitumour potential of the therapy using this type of virus and to identify the characteristics of those patients in which it works most effectively. The study will last for 3 years: 2 of which will be recruitment and treatment and 1 of which will be patient follow-up.
If the results are promising, it will be possible to open a new line of treatment for children diagnosed with this devastating disease. Unlike other more conventional treatments, the advantage of this treatment is its huge reduction in sequelae. This is due to its very mild side-effects and the fact that it does not damage brain tissue. A safe, targeted treatment with the potential to massively improve the quality of life of these children in the future is in sight.
Latest developments:
Despite the difficulties arising from the COVID-19 pandemic, which have delayed all of the administrative procedures, the team has successfully managed to undertake all of the necessary processes with the Spanish Agency of Medicines and the Drug Research Ethics Committee. All of these processes are essential to ensuring that the clinical trials, which involve people, comply fully with the safety requirements, provide patients with information and are well backed up by scientific evidence.
As a result of the team’s hard work and previous clinical trials with CELYVIR (which certify the safety of this treatment), it is anticipated that the processes will be concluded shortly and the trial will begin prior to the summer of 2021.