BRAS Drug Development Fellowship Program
ABOUT THE FELLOWSHIP
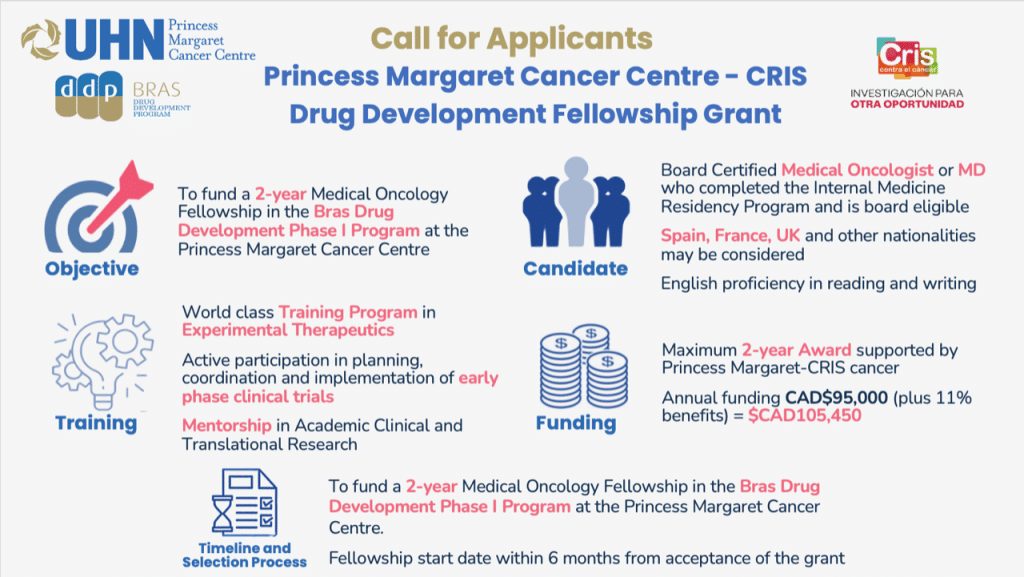
The BRAS Drug Development Fellowship Program is a world class training program in experimental therapeutics with a mentorship track record of over 50 trainees in the last two decades. The Fellowship, directed by Dr. Anna Spreafico, is part of the Drug Development Program at the Princess Margaret Cancer Centre, University Health Network (PM-UHN) and includes 4 to 8 clinical research fellows annually. This year, CRIS and the Phase I Clinical Trials Program PM-UHN are co-funding one fellowship candidate.
Purpose
The goal of the CRIS-PM Drug Development Fellowship is to train early career investigators from Spain, France and UK (and potentially other countries) at the PM-UHN in Toronto, Canada, in experimental therapeutics and translational research. Applicants to the CRIS-PM Drug Development Fellowship are expected to complete a two-years training in the Phase I Clinical Trials Program, following their board certification in Medical Oncology in Spain or another acceptable jurisdiction.
Eligibility
Applicants must meet the following criteria:
- Board Certified Medical Oncologist or MD who completed the Internal Medicine Residency Program and is board eligible.
- Spain, France or UK nationality (other countries may be considered).
- English proficiency in reading and writing
Terms and conditions
You can download the Terms and Conditions from this link
On 3rd April it was held a webinar in order to clarify any doubts that might arise in relation to these calls. To watch the video, go here
Application
Please submit your application to diana.Burgos@uhn.ca
Call for applications open until 30th June 2024