Background:
Children’s cancer has had no relevant treatment improvements in the past few years, in spite of its being the first cause of childhood death in our country. Treatments applied to adults are not suitable, as biologically these are very different tumours, which evolve more aggressively, cannot be prevented and have no early diagnosis strategies.
Furthermore, treatments applied to children are more intense than those applied to adults, and usually have more serious side-effects, as patients’ bodies are in growth stage and most therapies applied purport to destroy cells that multiply fast.
Project Description:
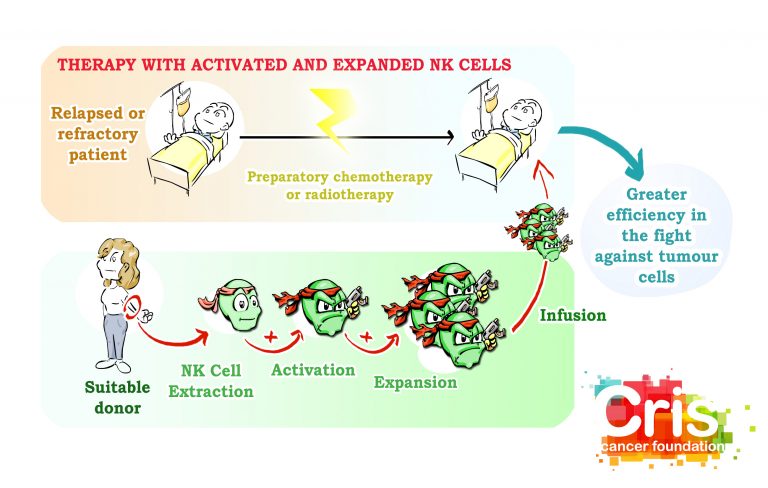
The Lydia trial is destined to improving the immune system of children with treatment-resistant acute leukaemia, or who have suffered a relapse. A therapy is applied consisting of infusing Natural Killer cells, which are experts at eliminating aberrant and tumour cells. Cells are extracted from the parents, are activated and multiplied and are introduced to the child, who receives exceptional reinforcements to fight against cancer.
Lydia is a Phase I trial, which attempts to establish whether these treatments are safe. If a Phase I trial is successful, the trial list continued to confirm the positive effects of the treatment in a Phase II trial.
Achievements in the past year:
The trial has been closed, with the recruitment of 20 patients. As a result of children abandoning the trial or progression or remission of the disease, the trial was finally performed on 18 patients. The results of the study had been excellent, as the goal was to assess the therapy’s toxicity, and the patients’ tolerance to the treatment has proved excellent. Furthermore, 7 patients achieved complete remission, and in 4 of them, no residual disease was detected. 10 patients received a bone marrow re-transplant to consolidate the therapy.
Currently, 4 patients have survived without relapse for more than a year and a half. This is excellent news because, with the passage of time, the likelihood of relapse or death is low. To summarise, 30% of patients without an available treatment or resistant to treatment have benefited from the therapy.
This has allowed us to launch the next phase of the trials, a Phase II trial called Lydia II, with the objective of preventing a relapse. The trial includes hospitals distributed throughout Spanish territory (Bilbao, Málaga, Madrid, Murcia and Badajoz), and patients are already being recruited. Some of the children have been able to return home, which makes us think that this trial will also be successful and that the therapy will continue on the path to becoming a conventional therapy.
.